Introduction
B-cell acute lymphoblastic leukemia (B-ALL) is the most common pediatric cancer. In recent years, our understanding of B-ALL biology has benefited from genomic and transcriptomic analyses that identify driver alterations in 98% of patients. In addition, prior studies of monozygotic twins, who both developed B-ALL, have provided insight into the developmental timing and natural history of the disease, as well as the identification of novel mechanisms of leukemogenesis.
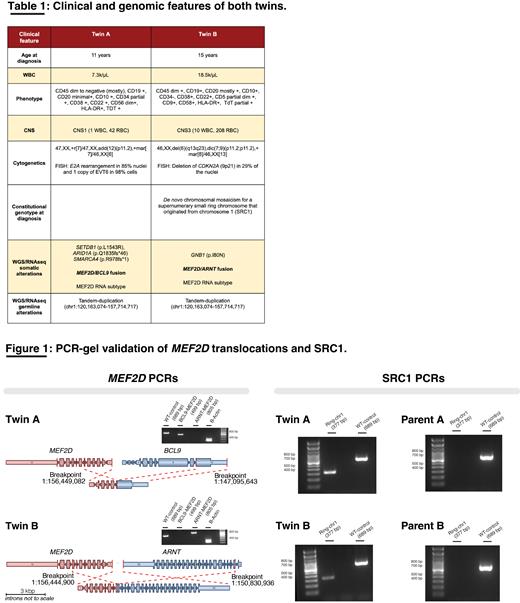
Herein we study a case of monozygotic twins, who developed B-ALL (Table 1). We report the characterization of a de novo supernumerary ring chromosome 1 (SRC1) as an initiator event that followed independent but partially convergent clonal trajectories in each twin. Thus, SRC1 resulted in genomic rearrangements of MEF2D in both twins but targeted distinct fusion gene partners ( BCL9 versus ARNT). To our knowledge, this study represents the first description of MEF2D-ARNT as an event involved in B-ALL, and the association of SRC1 Syndrome with leukemia.
Methods
We performed integrative whole genome sequencing (WGS) and RNAseq analysis on B-ALL samples at diagnosis and compared this to WGS data from minimal residual disease negative bone marrow aspirates. Coverage levels for tumors and matched normals were: Twin A=104 & 59; Twin B=81 & 53. Tumor purity estimations were 0.80 in Twin A and 0.31 in Twin B. For RNAseq, expression profile efficiencies were 61% and 36% respectively. Comprehensive variant calling was performed using the Isabl platform (Medina et al, 2020) to map all putative somatic and germline events. ALLCatchR (Beder et. al, 2022) was used for molecular B-ALL subtype allocation in 26 samples from our pediatric cohort of patients including the twins (age range 1-23), and MutationTimeR (Gerstung et al, 2020) to calculate the relative timing of copy number gains. All research studies were approved by NYU and MSKCC Institutional Review Boards.
Results
The tumor mutational burdens for each leukemia sample were 0.86 for Twin A and 0.47 mutations/Mb for Twin B, and they showed independent alterations in oncogenic genes (Table 1). The only B-ALL driver that both twins had partially in common was an intrachromosomal translocation resulting in MEF2D fusions, but each with a distinct partner gene ( BCL9 versus ARNT) which was confirmed by RNAseq (supporting reads: Twin A=48, Twin B=107) and PCR assays (Fig. 1). Each sample demonstrated enhanced transcription levels of MEF2D compared to our reference B-ALL cohort (transcripts per million: Twin A=48.0, Twin B=77.4, median=27.7, sd=19.9), and were transcriptionally classified as MEF2D-subtype by the ALLCatchR classifier. These results functionally supported the role of MEF2D-ARNT as a new oncogenic event.
Analysis of germline variants showed a tandem duplication flanking the pericentromeric region of chromosome 1 in both twins (1:120,163,074-157,714,717) where MEF2D BCL9and ARNT are localized, that had been previously identified as SRC1 in only one of them by constitutional genotyping (Table 1). Cell fractions in normal and tumor tissues were: Twin A=14.8% & 50%, Twin B=8.6% & 26.5%. PCR interrogation of parental germline DNA failed to identify SRC1, suggesting this to be a de novo event in the affected twins (Fig. 1).
We compared the relative timepoint in which the SRC1 took place in each sample using MutationalTimeR, but we were only able to obtain results in Twin A due to a higher mutational burden (number of variants in SRC1: Twin A=44, Twin B=13). As expected, the tandem duplication that formed the SRC1 was identified as an early mutational event (confidence interval of relative time of appearance: 0-0.4).
Conclusions
Our results identify SRC1 as the common initiating genomic event leading to MEF2D fusion driven B-ALL in both twins. These findings support a model in which a tandem duplication around a pericentromeric area was an initiating event which caused the scission and circularization of DNA without the loss of genetic material. This led to a de novo acquisition of a SRC1, that after independent but partially convergent clonal trajectories gave rise to 2 distinct oncogenic MEF2D fusion driven leukemias in each twin . Moreover, ARNT is a novel partner gene to MEF2D which has not been previously described.
This is the first report of B-ALL association with SRC1 Syndrome, which emphasises the utility of the study of monozygotic twins to identify novel alterations underlying B-ALL.
Disclosures
Gundem:Isabl Inc.: Consultancy. Levine:Isabl Inc.: Current Employment, Current holder of stock options in a privately-held company, Patents & Royalties. Medina Martinez:Isabl Inc.: Current Employment, Current equity holder in private company, Membership on an entity's Board of Directors or advisory committees. Kung:Isabl Inc.: Current Employment, Current equity holder in private company, Membership on an entity's Board of Directors or advisory committees. Shukla:Syndax Pharmaceuticals: Honoraria. Papaemmanuil:Isabl Inc.: Current equity holder in private company, Current holder of stock options in a privately-held company, Other: CEO, Patents & Royalties: Whole genome cancer analysis; TenSixteen Bio: Current equity holder in private company.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal